Ordained-Becoming
Technology is evolution, accelerated. In order to see where technology is speeding towards we need to understand where evolution has taken it so far.
Because this is a very long post (13,000 words — my blog software made me post it into two parts), let me tell where I end up so you can judge whether you want to come along: The course of evolution is not random. It has an inherent direction, shaped by the nature of matter, and this direction induces inevitabilities in the shape of life. These tendencies are extended into technology, which means aspects of the technium are also inevitable. My argument for technological determinism begins at the beginning.
Four billion years ago the first DNA molecule began ceaselessly replicating with occasional modification. In the four billennia since, DNA has modified itself uncountable times. The 100 billion separate species that are estimated to have lived on earth so far each represent only a tiny fraction of that prolific variation. Fifty thousand years ago this ever-busy little molecule unfolded the first conscious mind. Further uncountable variations over the last 50,000 years enabled this same molecule to produce one hundred billion human minds (the number of humans that have ever lived), which unfolded the millions of species of technology that now surround us. Even though in retrospect there are many discontinuities that appear to “change everything” along the way – human language being one example — day by day evolution spreads incrementally and without gaps. The path from the very first DNA molecule to the pixilated screen in front of you is one long continuous arc.
Every species of technological machinery operating today can trace its roots back in an unbroken line of variations to primeval life. Yet between the first variation of the double helix and the latest variation of the microscopic computing chips running the internet are many fossils. Literal fossils. Bits of weird creatures long extinct buried and squeezed between rocks. As we expose, catalog, classify, and analyze these preserved capsules of living variations, we are discovering a pattern of structured change that, in broad strokes, applies not only to organic life but to technology as well.
In 1974 Simon Conway Morris, a paleobiology graduate student at Cambridge University, began an intense study of obscure fossils hidden in an obscure location: a narrow outcrop of 500 million-year old shale crammed between two small peaks high up in the Canadian Rockies. The organisms he examined were very bizarre. There appeared to be nothing like them in the history of the life. One worm-like species, which he named Hallucigenia, as in hallucinogenic, was so unearthly bizarre that he concluded they walked on two parallel rows of needle spikes. Another organism had five eyes, and one sported a mouth with a circular row of teeth.
Conway Morris believed some of these long-gone species were outliers, exemplar specimens of phyla previously unknown to science. He cataloged not just one species, but dozens of hitherto unknown phyla – an entire underwater world of new body-designs for creatures that greatly expanded the known categories of animals. They were stunningly different in basic design from anything alive today. This layer of ancient life frozen in great detail by the fine-grained limestone dating from the Cambrian period later became known as the Burgess Shale fossils. Dissecting them was tedious work. The fossils were so minutely detailed, so weird, that he needed to tease apart their fragile remains with pin pricks under a microscope. But Conway Morris’s revelations began to overturn our understanding of evolution.
The wild disparity of the basic body designs of these ancient and long-gone creatures greatly outnumber the variety of animal forms we have now. It seemed as if life was more varied, more diverse long ago, and its choices have only narrowed since then. Conway Morris was only 23 years old when he started dissecting these curious fossils but in his youthful exuberance he believed they hinted at a new way to see the world.
Simon Conway Morris’s work on the Burgess Shale became the centerpiece of Steven J. Gould’s literary-science masterpiece Wonderful Life. To explain the emerging paradox of diversity, Gould introduced a metaphor in this book so elegantly stated that it is now unavoidable in biology. What if, Gould asked, we could rewind the tape of life? Would the story of evolution play out the same, or different? Gould mustered reams of paleobiological details to demonstrate that evolution is contingent on millions of lucky random forks in the road, and that if we re-run life we’ll get a different outcome each time. In particular, he suggests that the appearance of humans – our form, particularly our intelligence – is due wholly to unrepeatable chance. Replay evolution a thousand times and we’d never get anything close to a thinking ape. Or to put it in Gould’s masterful prose: “if we could perform the great undoable thought experiment of ‘rewinding the tape of life’ back to the Cambrian and ‘distributing the lottery tickets’ at random a second time, the history of animals would follow an entirely different but equally ‘sensible’ course that would almost surely not generate a humanoid creature with self-conscious intelligence.” (Natural History) In this view, absolutely nothing is inevitable in evolution. And since technology is an extension of evolution, we should not expect inevitabilities, or direction, in culture either.
Exhibit A in Gould’s argument for the inherent contingency of evolution were the ancient Burgess Shale fossils that Simon Conway Morris spent decades toiling over. These novel body designs were eradicated wholesale 530 million years ago. Only 35 out of several hundred basic designs from that time survived to become the foundational design for all later animals today. Had historical accidents been different, Gould argued, and the tape of life re-run, the basic designs pioneered in the Burgess shale animals could have survived, and much of what is alive today would be vastly different. We would not be here, or rather “we” might have an exoskeleton, or four arms, or another pair of eyes on the back of our head, if we even had a head.
This view of the fundamental contingency of evolution is now the orthodoxy in science. Every textbook on evolution today acknowledges the historical “lucky” aspect of evolution. A profound consequence of this contingency framework is that there can be no direction to evolution. There is no steady slow march toward higher complexity or anything else. There is no widening “cone of diversity” expanding outward into time. In fact, Gould argues, the cone of diversity is reversed, reducing the range of innovation (or disparity) as time passes. Broadly unique morphological designs (which Conway Morris and others believed they had found in the Burgess Shale) will sometimes be eliminated not because they are unfit (as usually happens in natural selection), but because an accidental perturbance, such as an asteroid hit, or extreme climate change, removes them for no more reason than pure bad luck. In those serendipitous accidents there is no chance for natural adaptation to work. In a truly contingent world, diversity is eliminated in a lottery fashion, forcing evolution to follow less optimal possibilities. Thus evolution ricochets around aimlessly without advancement.
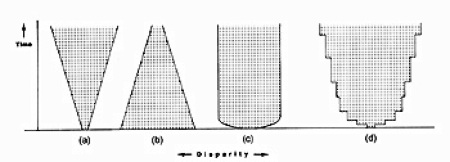
Possible trends in disparity by Simon Conway Morris. A is greater, B is diminishing, C is constant, and D is step-wise gain.
We only think there is a swelling rise of increasing complexity because our brains are hardwired to find patterns, so we tend to see trajectories anywhere we look. Therefore the apparent evolution of life towards greater complexity is a mere illusion. As Gould explains in another brilliant metaphor, a drunken man wandering aimlessly away from a wall will sooner or later fall into a ditch. Not because some force pushed him toward the ditch but because he can’t walk through the wall, so he is free to move in one general direction only, making his eventual arrival at the ditch statistically expected. In biology, the wall is simplicity (primitive organization), and the ditch is complexity. The first bacterium can’t get any simpler than it already is so it must randomly try things that are more complex and so it drifts away from the wall of ultimate simplicity. Evolution is the drunk that must stagger around until it falls into complexity. Life’s trend, if we want to call it that, is aimless randomness.
Convergences
However, today there is an emerging view running contrary to Gould’s magisterial claims of orthodoxy. The contrarians say that our intuitive sense of evolution is true, and it really has, without illusion, moved toward greater complexity and diversity over its grand sweep. Continuing Gould’s metaphor, in the contrary view the ground between the wall and ditch is tilted. An almost imperceptible slope carries life away from the simple and towards the more complex and diverse. Wherever on the slope of evolution an organism sits, it will tend to slip toward more complexity, however slightly. Over time that mild tilt supplies evolution with a decided direction.
But wait! This raises a whole bunch of alarming questions: If evolution has a direction, does it have a destiny? What is steering this tend, and where does this force reside? And does this mean that life repeats itself if you re-run it, so that aspects of life are inevitable?
Inevitable! Now there’s a word we don’t find in textbooks anywhere.
Can anything be inevitable in a free-willed world? The same currents that govern evolution also govern its seventh kingdom, the technium. If the evolution of life has a direction, than the evolution of technology must as well, since the technium sped out of evolution only recently and still depends on its biological parents (us). This ignites a similar set of even more alarming questions: If technological progress has a direction, where is it headed? Where does this guiding force reside, if not in us? And not least, are some technologies inevitable? If so, which ones?
In other words, if the ground of the technium is sloped to impart a bias to the advance of technology, then where does technology want to go? What does it want? We dare ask such questions only if life’s evolution is bent in certain directions. Evidence that replaying the tape of life produces similar designs would give us permission to ask the same questions in technology. If we re-ran the evolution of the technium would the exact same technologies occur again and again?
We can only begin to answer such a question by beginning with the essential antecedent question: is there any such evidence that the morphological forms of life are inevitable?
In tracing the origins of his conclusion that there is no direction to evolution Gould said, “I developed my views on contingency and the expanded range of Burgess diversity directly from Conway Morris’s work and explicit claims.” (web) How great the irony then that the scientist who has so far amassed the most evidence against the orthodoxy of contingency, and has emerged as the major spokesman for the view that evolution is full of inevitabilities is none other than Simon Conway Morris.
Conway Morris did what scientists are supposed to do, but rarely do: he changed his mind. Further work by other paleontologists on the species that Conway Morris enthusiastically heralded as wildly new entrants into the flux of life demonstrated that they were misidentified. His Hallucigenia was not a new kind of worm walking on spikes but a weird old worm with common legs and spikes down his back. “We made some mistakes,” he says. “With the benefit of hindsight, we can see that we had exaggerated the diversity of these supposedly bizarre fossils and needed to reconsider their evolutionary relationships.” In many cases the unearthly alien creatures of the Burgess Shale turned out to be new species in old familiar lineages. Their inclusion did widen the diversity of the existing categories, but they forced no radically new categories.
But it was the publication of Gould’s majestic book about his own work that first planted a seed of doubt in Conway Morris’s mind. With his own expert paleontological eyes, Conway Morris found that the scientific examples that Gould used for contingency could also be interpreted in the opposite way — just as he himself mistakenly described Hallucegenia as belonging to a new phyla when he actually had the organism upside down! The more he looked at the evidence of historical contingency the more he saw evidence for inevitabilities. His investigations into the deeper structure of evolution led him to eventually write, “Everything we know about biology argues that it is seeded with inevitabilities.”
Inevitable Forms Most Beautiful
When Charles Darwin was working out his theory of natural selection, the eye worried him. He found it very hard to explain how it could have evolved bit by bit, because the eye’s retina, lens, and pupil seemed so finely perfected toward the whole, and so utterly useless at less than whole. Critics of Darwin’s theory of evolution held the eye out as a miracle. But miracles, almost by definition, happen only once. Neither Darwin, nor his critics, appreciated the fact that the camera-like eye evolved not just once – miracle though it may seem – but six times over the course of life on earth. The remarkable optical architecture of a “biological camera” is also found in certain octopus, snails, marine annelids, jellyfish, and spiders. These six lineages of unrelated creatures share only a distant camera-eyeless common ancestor, so each lineage gets credit for evolving this marvel. Each of the six manifestations is an astounding achievement; after all it took humans several thousands of years of serious tinkering to cobble together the first artificial one.
But does the six-time independent self-assembly of the camera eye signal a supreme degree of improbability, sort of like tossing 6 million penny-heads in a row? Or does the six-time invention mean that the eye is a natural funnel that attracts evolution, like water in a well at the bottom of a valley? And then there are the 8 other types of eyes, each of which has been evolved more than once. Biologist Richard Dawkins estimates that “the eye has evolved independently between 40 and 60 times around the animal kingdom,” leading him to claim, “It seems that life, at least as we know it on this planet, is almost indecently eager to evolve eyes. We can confidently predict that a statistical sample of [evolutionary] reruns would culminate in eyes. And not just eyes, but compound eyes like those in an insect, a prawn, or trilobite, and camera eyes like ours or a squid’s…. There are only so many ways to make an eye, and life as we know it may well have found them all.”
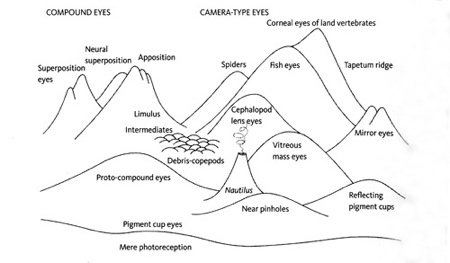
Landscape of possible eye forms by Michael Land.
Are there certain forms – “natural” states — that evolution tends to gravitate towards? Millions of experiments done on computers show that complex adaptive systems, such as evolution, tend to settle (all other factors begin equal) into a few recurring patterns, known in mathematics as “attractors.” Even though the system may start out in different initial states, if one runs the program enough times, it tends to attract itself to a few repeating patterns. These patterns are not found in the parts and so the structure that appears is considered both “emergent” and dictated by the complex adaptive system. Since the same structure will appear again and again – like a vortex in a draining tub – they are also considered inevitable.
In the bottom drawer of their desks biologists have long held an ever-growing list of cases of identical phenomenon that have appeared more than once on earth. These curious cases – sort of vortexes in the river of life — are usually filed and forgotten. But a few scientists believe they are biological attractors. The 30-100 million species presently co-inhabiting earth are running millions of experiments every hour. Out of this exhaustive recombination, constant tweaking, and ceaseless interaction the complex adaptive system of evolution keeps converging upon similar characteristics in far flung branches in the tree of life. This attraction is called convergent evolution. The best of these examples show that highly adaptive designs can originate independently in separate lineages. The more taxonomically separate the lineages, the more impressive the convergence.
Old World primates have full color vision and inferior smell compared to their distant second-cousins the New World monkeys. These spider monkeys, lemurs, and marmosets all have a very keen smell but lack tricolor vision. All, that is, except for the howler monkey, who in parallel to the Old World primates, has tricolor vision and a weak nose. The common ancestor to the Howler and the Old World primates goes very far back, so howlers independently evolved tricolor vision. By examining the genes for full color vision, biochemists discover that both Howler and Old World primates used receptors tuned to the same wavelengths, and they contained exactly the same amino acids in three key positions. Not only that, the diminished olfactory senses of howler and apes was caused by the inhibition of the same olfactory genes, turned off in the same order, and in the same details. “When similar forces converge, similar results emerge. Evolution is remarkably reproducible,” says geneticist Sean Carroll.

List of convergent evolution compiled by Connie Barlow.
Depending on how one measures the concept of “independent,” the catalog of visible examples of independent convergent evolution is hundreds long, and counting. Any list will certainly include the three-time evolution of flapping wings in birds, bats and pterodactyl (reptiles of the dinosaur era). The last common ancestor among these three lineages did not have wings, which means that each evolved their wings independently. Despite their vast taxonomic distance, the wings in each of these three cases are remarkably similar in form: skin stretched over bony limbs. Navigation by echo-location has been found four times in bats, dolphins and two species of cave-dwelling birds (the South American oilbird and Asian swiflets). Bipedality recurs in humans and birds. Anti-freeze compounds were evolved twice in ice-fish, once in the Artic and once in Antarctic. Both humming birds and sphinx moths evolved to hover over flowers sucking nectar through a thin tube. Warm-bloodedness evolved more than once. Binocular vision many times in distant taxon. Bryozoa, a family of coral, evolved distinctive helical shaped colonies six different times over 400 million years. Social cooperation evolved in ants, bees, rodents, and mammals. Seven widely separated corners of the plant kingdom evolved insectivorous species – eating insects for nitrogen. Succulent leaves multiple times evolved across taxonomic distance. Jet propulsion twice. Buoyant swim bladders evolved independently in many varieties of fish, mollusks, and jellyfish. Flapping wings constructed of taunt membranes over skeleton frames arose more than once in the insect kingdom. While humans have technically evolved fixed-wing aircraft and spinning wing aircraft, we haven’t yet made a viable flapping wing craft. On the other hand, fixed wing gliders (flying squirrels, flying fish) and spinning wing gliders (many seeds) have evolved a number of times. In fact, three species of rodent-like gliders also display convergence: the Flying Squirrel, and the Squirrel Glider and the marsupial Sugar Glider, both of Australia.
Because of its lone tectonic wanderings in geologic time, the continent of Australia is a laboratory for parallel evolution. There are multiple examples of marsupials in Australia paralleling placental mammals from the old world. Even in the past. Saber-canine teeth are found in both the extinct marsupial thylocosmilid and the extinct saber tooth cat. Marsupial lions had retractable claws like feline cats.
Dinosaurs, our iconic distant cousins, independently evolved a number of innovations in parallel with our common vertebrate ancestors. In addition to the parallels between flying Ptetrodactyls and bats, there were the streamlined Ichthyosaurs that mirrored dolphins, and Mosasaurs which paralleled whales. Triceratops evolved beaks similar to both parrots and octopus and squid. Snake-like Pygopodidae were as legless as reptilian snakes later were.
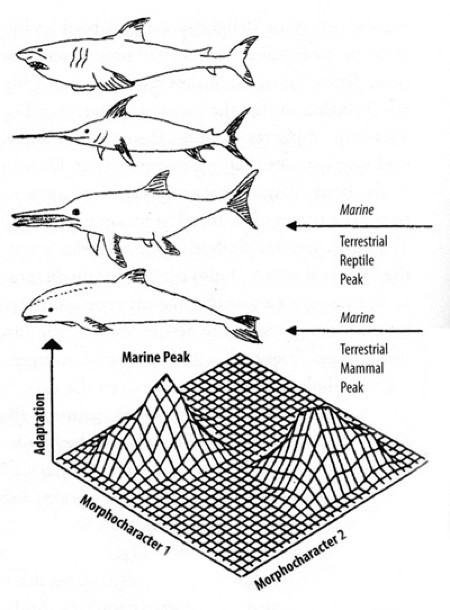
Convergence of streamline shape.
The less taxonomic distance between lineages, the more common, — but less significant – convergence becomes. There are five unrelated species of dolphins that live only in rivers. Both frogs and chameleons independently evolved rapid-fire “harpoon tongues” to snatch prey at a distance. All three major phyla of mushrooms have separately evolved species that produce dark, dense, underground, truffle-like fruits; and in North America alone there are more than 75 genera that include “truffles,” many of which evolved independently.
For some biologists occurrences of convergence are merely a statistical curiosity. Sort of like meeting someone else with your own name and birth date. Weird, but so what? Given enough species enough time you are bound to encounter two that cross paths morphologically. But homologous features are actually the rule in biology. Most homology is invisible, and among related species. Related species naturally share features, unrelated less so, so unrelated homology is more meaningful and noticeable. Either way, most methods used by life are used by more than one organism, and in more than one phyla. What is rare is a trait that has not been re-used somewhere in nature. Richard Dawkins challenged naturalist George McGavin to name “innovations” that have evolved only once, and he was able to compile only a handful, such as the bombardier beetle that mixes two chemicals on demand to shoot a noxious stream at enemies, or the diving bell spider, which uses a bubble to breathe.
Return to the returning eye. The eyeball retina is lined with a layer of a very specialized protein that performs the tricky work of perceiving light. This protein, called rhodopsin, transfers the photon energy from incoming light to an outgoing electrical signal sent along the optic nerve. Rhodopsin is an archaic molecule present not only in the retina of camera-eyes, but also in the most primitive lensless eye-spot of a lowly worm. It is found throughout the animal kingdom, and it retains its structure wherever it is found because it works so well. The same molecule has probably remained unchanged for billions of years. Several competing light-trigger molecules (crytochromes) aren’t as efficient, or robust, suggesting that rhodopsin is simply the best molecule for seeing that can be found after 2 billion years of looking. But surprisingly, rhodopsin is another example of convergent evolution, because it evolved twice in two separate kingdoms in the deep past. Once in Archaea and once in Eubacteria.
This fact should shock us. The number of possible proteins is astronomical. There is an alphabet of 20 base symbols (amino acids), which make up every protein “word” which on average is say, 100 symbols, or 100 bases, long. (In fact many proteins are much longer, but for this calculation 100 is sufficient.) The total number of possible proteins that evolution could generate (or discover) is 100^20 or 10^39. This means that are more possible proteins than there are stars in the universe. But let’s simplify that. Because only one in a million “words” fold into a functioning protein, let’s vastly reduce that magnitude and agree that the number of potential working proteins is equal to the number of stars in the universe. Discovering a specific protein would be equal to arriving at a specific star.
By this analogy evolution finds new proteins (new stars) by a series of hops. It jumps from one protein to a “nearby” related one, and then hops onto the next novel form until it reaches some remote unique protein far from where it started, just as one might travel to a distant sun by hopping stars. But in a universe as large as ours, once you landed on a distant star one hundred hops away, you would never reach it again by the same random process. It is statistically impossible. But that is what evolution did with rhodopsin. Out of all the protein stars in the universe, it found this one – a protein that has not been improved upon for billennia – twice.
But the impossibility of “twice-struck” keeps happening in life. Evolutionist George McGhee writes in Convergent Evolution: “The evolution of the ichthyosaur or porpoise morphology is not trivial. It can be correctly described as nothing less than astonishing that a group of land-dwelling tetrapods, complete with four legs and a tail, could devolve their appendages and their tails back into fins like those of a fish. Highly unlikely, if not impossible? Yet it happened twice, convergently in the reptiles and the mammals, two groups of animals that are not closely related. We have to go back in time as far as the Carboniferous to find a common ancestor for them; thus, their genetic legacies are very, very different. Nonetheless, the ichthyosaur and the porpoise both have independently re-evolved fins.”
What then guides this return to the improbable? If the same protein, or “contingent” form, is evolved twice it is obvious that every step of the way cannot be random. The prime guidance for these parallel journeys is their common environment. Both archaea rhodopsin and Eubacterial rhodopsin, and ichthyosaur and dolphin, float in the same seas with the same advantages gained by adaptations. In the case of rhodopsin, because the molecular soup surrounding the precursor molecules is basically the same, their selection pressure will tend to favor the same direction on each hop. In fact, the match of environmental niche is usually the reason given for most occurrences of convergent evolution. Arid, sandy deserts on different continents tend to produce large-eared, long-tailed, hopping rodents because the climate and terrain sculpts a similar set of pressures and advantages.
Yes, but why then doesn’t every similar desert in the world produce a Kangaroo Rat, or Jerboa, and why aren’t all desert rodents Kangaroo Rats? The orthodox answer is that evolution is a highly contingent process, where random events and pure luck change the course, so that even within parallel environments it is very rare to arrive at the same morphological solution. Contingency and luck are so strong in evolution that the marvel is that convergence ever happens. Based on the number of the possible forms that can be assembled from the molecules of life, and the central role of random mutation and deletion in shaping them, significant convergence from independent origins should be as scarce as miracles.
But a hundred, or thousand, cases of isolated significant convergent evolution suggest something else at work. Some other force pushes the self-organization of evolution towards recurring solutions. A different dynamic besides the lottery of natural selection steers the course of evolution so that it can reach a remote unlikely destination more than once. It is not a supernatural force, but a fundamental dynamic as simple in its core as evolution itself.
Evolution is driven toward certain recurring and inevitable forms by two forces of convergence:
1) The negative constraints cast by the laws of geometry and physics, which limit the scope of life’s possibilities. And,
2) The positive constraints produced by the complexity of interlinked genes and metabolic pathways, which generates a few repeating new possibilities.
These two dynamics create a push in evolution that gives it a direction. Both of these forces continue to operate in the technium as well. The two dynamics shape the inevitabilities of technology. Let me address each biological influence in turn.
The Negative Constraints of Matter
Life – even in the most alien alternative we can possibly imagine – requires flexibility. Material flexibility requires chemical bonds that switch easily, electrons that can be caught or sent easily, molecules that can dissolve and precipitate without great energy expenditures. This ease of change is essential for life, and the matter in our universe is just right for self-organized change. For reasons we don’t understand, the laws of the universe contain many goldilocks zones, with settings that are “not too little, not too much” for flexibility and life. Just six basic cosmological constants govern the dimensions that enable extropic, self-organizing structures in the universe. If those values settled at even a minute difference away from the values they now have this universe would not contain stars, galaxies, planets, or much of the physical organization of matter and energy that we know. A further set of some 30 cosmological constants are sympathetic towards life as we know it.
But every single one of these favorable goldilocks constants also significantly shapes what is built under their sway. For example water molecules in aggregate possess a set of peculiar characteristics. Water can hold and transport myriad other molecules, it can form a crystalline structure less dense than its liquid form, it is transparent in visible spectrums, and it wields a polar charge and high surface tension. All of water’s just-right goldilocks qualities influence, however indirectly, everything made with water. Ditto for other elements and energetic forces; their quirks ripple outward. The constraints of H2O and oxygen and carbon trickle up into molecules constructed with them, and eventually their constraints indirectly govern even the organisms made with those molecules.
One example: Plants and animals come in a bewildering diversity of scales. Insects can be microscopic like lice, or giant, like horned beetles the size of shoes; redwood trees tower 100 meters tall, and miniature alpine plants fit in a thimble; immense blue whales swell as big as ships, and pygmy chameleon shrink to less than an inch long. Yet the size of each species of these plant and animal is not arbitrary. They follow a law that is astonishingly constant, dictated by the physics of matter: the mass of an organism scales to the third power of its body length. The surface tension of water, ordained by the structure of H2O, dictates the strength of a cell wall, which mandates the maximum height per width, which constrains the form. The size of a creature, therefore is linked to its mass and vice versa and no plant or animal wavers far from this constant slope. These physical forces play out not just on earth, but everywhere in the universe, and so we might expect any organisms based on water, whenever and wherever they evolve, to converge upon this same universal size ratio (adjusted for local gravity).
The metabolism of life is likewise constrained. Small animals live fast and die young. Big animals plod along. The speed of life for animals – the rate at which their cells burn energy, the speed their muscle twitches, the time it takes them to gestate, or to mature – is remarkably proportional to their life span and size. It turns out metabolic rate is proportional to mass to the 3/4 power, and its heart rate is proportional to mass to the –1/4 power. These constants derive from the fundamental rules of physics and geometry, and the natural advantages to minimize energy surfaces (lung surface, cell surface, circulatory capacity, etc). While a mouse’s heart and lungs beat rapidly compared an elephant’s, both mouse and elephant count the same number of beats and breaths per life. It is as if mammals are assigned 1.5 billion heartbeats, and told to use them as you like. Tiny mice speed ahead in a fast-forward version of elephant life.
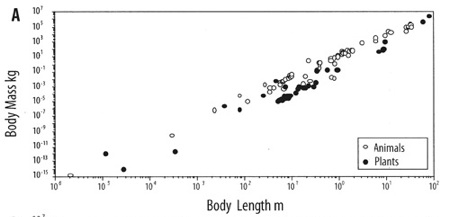
The even slope of mass/length in both animals and plants.
In biology this power law was well known for mammals, but researchers recently realized a similar law governs all plants, bacteria and even ecosystems. By factoring in the “body” temperature of the physical process as well as its density, the ratio of energy use, mass, and size all converge toward a constant rate that is a multiple of a 1/4 exponent. Dilute soups of cool oceanic algae are slo-mo versions of life packed dense in a warm-blooded heart. Many living processes — from the number of hours of an sleep an animal needs, to hatching times for eggs, to the rate at which a forest accumulates wood mass, to the mutation rate in DNA — all seem to follow this universal scaling law. “We’ve found that despite the incredible diversity of life, from a tomato plant to an amoeba to a salmon, once you correct for size and temperature, many of these [metabolic] rates and times are remarkably similar,” say Gillooly and West, the researchers who discovered this law. “Metabolic rate is the fundamental biological rate” they claim – “a universal clock” reckoned in energy, at which all life proceeds.
Other physical constants run through the biological world. Bilateral symmetry recurs in almost every family of life. It seems to bring adaptive advantage on many levels, from balance to redundancy to compression of code. Other geometric forms, like a tube for transport in plants or animals, or legs, are just plain good physics. Some recurring designs, such as the arboreal splay of branches in a tree and coral, or the swirling spiral of petals on a flower are based on the mathematics of growth. They repeat because the math is eternal. Biochemists Michael Denton and Craig Marshall state that “recent advances in protein chemistry suggest that at least one set of biological forms — the basic protein folds — is determined by physical laws similar to those giving rise to crystals and atoms. They give every appearance of being invariant platonic forms.”
There are some branches of life that increase the frequency at which they recycle evolutionary solutions (called homoplasy, or non-independent convergence) as they diversify, almost as if the pool of possibilities in that neck of the woods was becoming exhausted. Conway Morris observes that “evolutionary novelty is often only skin deep because it relies more on co-option and redeployment than invention.” For a taxonomic branch stuck in redeploying the same tricks it usually takes a discontinuous radical evolutionary breakthrough to shake up the family tree and generate novel solutions again.

The imaginary Groveback by Wayne Barlowe.
A “periodic table” of existing life forms graphed on a matrix of physical characters would reveal blank white spaces lacking organisms that “could be.” Such “could be” life forms that obey the constraints of matter – because we see the same form in other taxon — include a mammalian snake, a dinosaur mole, a flying spider, or a terrestrial squid. In fact, some of these could still evolve on earth, if we left the current flora and fauna alone long enough. (See Dougal Dixon’s magical “Zoology of the Future” in “After Man”) These speculative creatures are entirely plausible because they are convergent, recycling (but remixing) morphological forms that repeat throughout the biosphere.

An ET angler fish by Boulay and Steyer.
When artists and science-fiction authors fantasize alternative planets full of living creatures, try as they might to “think outside the box” of earthly constraints, many of the organisms they envision also retain many of the forms found on Earth. Some would chalk this up to a lack of imagination; we are constantly being surprised by bizarre forms found in the deepest part of the oceans on our own home planet; surely life on other planets will be full of surprises. Others, myself included, agree that we will be surprised, but that given what “could be” – that vast imaginary space of all possible ways in which one could make an organism – what we will find on another planet will only fill one small corner of what could be. Life on other planets will be surprising because of what it does with what we already know. Biologist George Wald, who won a Nobel prize for his work on eye retina pigments told NASA, ” I tell my students: learn your biochemistry here and you will be able to pass examinations on Arcturus.”
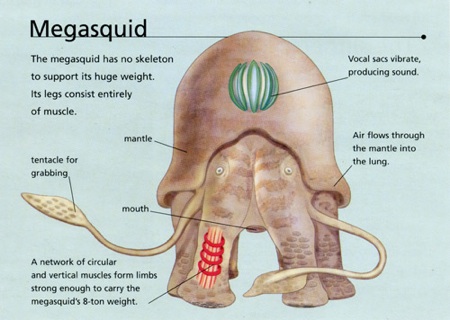
Dougal Dixon’s reasonable terrestrial Megasquid.
Any wannabe worldbuilder intent on birthing a consistent alternative biology should immerse themselves in the 1,100 pages of D’Arcy Wentworth Thompson’s classic study “On Growth and Form.” In overflowing detail this in-depth analysis demonstrates how the nature of materials and geometry governs morphology across nearly every taxon. Thompson examines the growth of mollusk shells, crustacean carapace, mammal skulls and skeletons, fish shapes, the venation of insect wings, the radial patterns of diatoms, and the maximal packing patterns of leaf cells, for just a few examples. Written in 1917, long before the Burgess Shale fossils were re-interpreted, Thompson concludes in the epic’s final pages that “the infinitude of possible forms is always limited,” and that “plain alternatives, of physico-mathematical possibility, are likely to repeat themselves.”
D’Acy Thompson’s laws of morphing.
Nowhere is that physical constraint of the infinitude more evident than in the structure of DNA. The molecule of DNA is so remarkable, it is in its own class. As every student knows, DNA is a unique double helical chain that can zip and unzip with ease, and of course replicate itself. But DNA can also arrange itself into flat sheets, or into interlocking rings, or even an octahedron. This singular gymnastic molecule serves as a dynamic mold that prints the stupendously large set of proteins responsible for the physical characterizes of tissue and flesh, which in turn, by mutual interaction, generates ecosystems of complexity. From this single omnipotent quasi-crystal the awesome variety of life in all its unexpected shapes springs forth. From subtle rearrangements along its tiny ancient spiral DNA projects the majesty of a strolling sauropod 60 feet high, the delicate gem of a iridescent green dragon fly, the frozen immaculacy of an white orchid petal, and of course the intricacies of the human mind.
If we acknowledge no supernatural force working outside of evolution, then all these structures – and more – must in some sense be contained within the structure of DNA. Where else could they come from? Their possibility must be held in the greater potential of this remarkable crystal. In the same way that the filigreed details, distinctive branching, furrowed bark, and lobed leaves of a white oak tree are all contained in its acorn, the details of all oak lineages and future species of oaks are resident, in some fashion, in the original acorn of DNA. “Some potentially useful mutations are so probable that they can be viewed as being encoded implicitly in the genome,” says biologist L. H. Caporale.
Of course merely inspecting this molecule reveals none of this cornucopia; we seek in vain to find a giraffe in the spiral ladder of DNA. But we can seek alternative “acorn” molecules as a way to re-run this unfolding to see if something else besides DNA could also generate similar diversity, reliability, and evolvibility. A number of scientists have searched for alternatives to DNA in the laboratory by engineering “artificial” DNAs, or constructing DNA-like molecules, or by engineering wholly original biochemistry. There’s a bunch of practical reasons to wield a DNA alternative, but so far alternatives with DNA’s versatility and brilliance are in short supply.
The first obvious approach in the quest for an alternative DNA molecule is to substitute slightly modified base pairs into the helix. K.D. James and A. D. Ellington write in “Origins of Life and Evolution of the Biosphere” that “experiments with alternative base pairing schemes have suggested that the current set of purines and pyrimidines [the canonical base pair types] is in many ways optimal…the unnatural nucleic acid analogues that have been examined experimentally have proven to be largely incapable of self-replication.”
Of course science is rife with discoveries initially thought unlikely, implausible, or impossible. In the case of self-organizing life, we might want to be particularly hesitant to generalize about alternatives since everything we can say about it is based on a sample size (so far) of exactly one.
But chemistry is chemistry, everywhere in the universe. Carbon sits at the center of life because it gregarious and contains so many hooks for other elements to bind to. It has a particularly friendly relationship with oxygen. Carbon is easily oxidized as fuel for animals, and easily un-oxidized (reduced) by chlorophyll in plants. And of course it forms the backbone for long chains of incredibly diverse mega-molecules. Silicon, carbon’s sister element, is the most likely alternative to produce a non-carbon-based life form. Silicon also is very prolific in its hooking up with all variety of elements and it is more abundant on the planet than carbon. But silicon suffers from a few major drawbacks. It does not link up into chains with hydrogen, limiting the size of its derivatives. Silicon-silicon bonds are not stable in water. And when silicon is oxidized, its respiratory output is a mineral precipitate, rather than the gas like carbon dioxide. That makes it hard to dissipate. A silicon creature would exhale bricks of sand. Basically silicon produces dry life. Without a liquid matrix it’s hard to imagine how complex molecules are transported around to interact. Perhaps silicon-based life inhabits a fiery world and the silicates are molten. Or perhaps the matrix is very cold liquid ammonia. But unlike ice, which floats and insulates the unfrozen liquid, frozen ammonia sinks, allowing the oceans to freeze whole. These concerns are not hypothetical, but are based on experiments to produce alternatives to carbon-based life. So far, all evidence points to DNA as the “perfect” molecule.
For even though clever minds like ours may invent a new lifebase, finding a lifebase which can evolve itself is an entirely higher order. The synthetic lifebase we create in the lab may be robust enough to survive on its own in the wild. But an alternative synthetic life does not need to self-organize itself into existence. That after all, is the whole purpose of minds. Minds create things that don’t need to be self-born. If you can skip the need of a self-made birth, you can jump to all kinds of complex systems that would never evolve on their own. Minds liberate types of complexity that evolutionary origins prevent. Robots and AIs don’t need to self-organize from metal-laden rocks.
However, DNA did. By far the most amazing thing about the strangest molecule in the universe is that this potent nucleus of life put itself together. The most basic carbon-based ingredients – such as methane or formaldehyde — are readily available in space, and even in pools on planets. But every abiotic condition (lightening, heat, warm pools, impact, freezing/thawing) we have tried as a stimulus to organize these lego-like building blocks into the elementary sugars of RNA and DNA fail to generate sustainable amounts of them. In experiments to artificially generate the components of a key sugar such as ribose (the R in RNA, and the ribo in deoxyribonucleic acid, DNA), the ribose is swamped by scores of other compounds in large quantities, which tend to degrade the small amount of ribose. All the known pathways to creating ribose are so complicated they are difficult to reproduce in the lab and (so far) unthinkable as existing in the wild. And that is just for one of eight sugars. The necessary – and potentially contradictory — conditions to nurture dozens of other unstable compounds towards self-organization have not been found.
Yet, here we are, so we know that these peculiar pathways can be found. At least once. But the supreme difficulty of simultaneous improbable pathways working in parallel suggests that there may be only one molecule that can negotiate this maze, and self-assemble its scores of parts, self-replicate once birthed, and then unleash from its seed, the head-shaking, eye-popping, mind-blowing variety and exuberance we see in life on earth. It is not enough to find a molecule that can self-replicate and self-generate ever-larger mounds of increasing complexity. There may indeed be multiple amazing chemical nuclei capable of that. Rather the challenge is finding one that does all that and can make itself, too.
So far, there are no other contenders even close to offering that kind of magic. This is why Simon Conway Morris calls DNA “the strangest molecule in the universe.” And why, if it is true, that Norman Pace says there may be a “universal biochemistry” based upon it. He speculates: “It seems likely that the basic building blocks of life anywhere will be similar to our own, in the generality if not in the detail. Thus the 20 common amino acids are the simplest carbon structures imaginable that can deliver the functional groups used in life… Similarly, the five-carbon sugars used in nucleic acids are likely to be repeated themes… Further, because of the unique abilities of purines and pyrimidines to interact with one another with particular specificity, these subunits too, or something very similar to them, are likely to be common to life wherever it occurs.” To paraphrase George Wald: If you want to study ET, study DNA.
There is another hint of the unique (perhaps universally unique) power of DNA. Two molecular biologists (Freeland and Hurst) computationally generated random genetic code systems by substituting all possible single-nucleotide for all codons. Since the combinatorial sum of all possible genetic codes overwhelms the time in the universe to compute them, the researchers sampled a subset of these, focusing on those systems they classified as chemically viable. They explored a million variations (out of what they estimated to be a pool of 270 million viable alternatives) and ranked the systems on how well they minimized errors. After a million runs the measured efficiency of the genetic codes fell into a typical bell curve. Far off to one side was Earth’s DNA. Out of a million alternative genetic codes, our current DNA scheme was “the best of all possible codes,” they conclude, and even if it is not perfect, it is at least “one in a million.”
On the other hand, biologist Harold Morowitz sorted through a database of 3.5 million organic molecules with simple criteria to search for alternative intermediates for the formation of citrates – a candidate for the universal biochemistry of life. He found the same 11 compounds that evolution arrived at and 142 other possibilities that might work, although no one has tested them yet. This set of alternatives is relatively small (out of 3.5 million), suggesting that while life’s solutions may not be unique, they are limited and not at all infinite. As Morowitz notes, “There are only four different kinds of one-carbon compounds.” This narrow set severely constrains what can be built with these elementary blocks.
Chlorophyll is another strange molecule. It is ubiquitous on the planet, yet not optimal. The spectrum of the sun peaks in the yellow frequency, yet chlorophyll is optimized for red/blue color. As George Wald notes, chlorophyll’s “triple combination of capacities” — a high receptivity to light, ability to store the captured energy and relay it to other molecules, and its ability to transfer hydrogen in order to reduce carbon dioxide — made it essential in the evolution of solar gathering plants “despite its disadvantageous absorption spectrum.” Wald goes on to speculate that this non-optimization is evidence that there is no better carbon-based molecule for converting light into sugar, because if there were, wouldn’t several billion years of evolution produce it?
(It may seem like I contradict myself when I point out convergence via rhodopsin’s maximum optimization and chlorophyll’s unoptimization. But I don’t think the level of efficiency is central. In both cases it is the paucity of alternatives that is the strongest evidence for inevitability. In chlorophyll’s case, no alternatives appear after billions of years in spite of its imperfection, and in rhodopsin’s case, despite a few minor competitors, the same molecule was found twice in an otherwise vast empty field.)
The mind is a powerful tool. No doubt someday researchers in the lab will devise an alternative base to organic DNA that is able to unleash a river of life. Accelerated vastly, this synthetic lifebase might evolve all kinds of creatures, including sentient beings. However, this alternative living system — whether based on silicon, carbon nano-tubes, or nuclear gases in a black cloud — would have its own inevitabilities, channeled by the constraints embedded in its original seeds. It would not be able to evolve everything, but it could produce many types of life that our life could not. Some science fiction authors have playfully speculated that DNA might itself be such an engineered molecule. It is, after all, ingeniously optimized, over-engineered, and its origins are vexing. Perhaps DNA was cleverly crafted by superior intelligences, and shot-gunned into the universe to naturally seed empty planets over billions of years? We would be just one of many seedlings that sprouted from this generic starter mix. This kind of engineered panspermia might explain a lot, but it does not remove the uniqueness of DNA. Nor does it remove the channels that DNA has laid for evolution on earth.
The restraints of geometry govern life. “Underlying all the diversity of life is a finite set of natural forms that will recur over and over again anywhere in the cosmos where there is carbon-based life,” claim biochemists Michael Denton and Marshall. Evolution simply can not make all possible proteins, all possible light gathering molecules, all possible appendages, all possible means of locomotion, all possible shapes. Life, rather than being boundless and unlimited in every direction, is bounded and limited in so many directions by the bounded nature of matter itself.
We have every reason to marvel at the inventiveness and exuberance of evolution. Every day field biologists discover another organism on earth, or something new about a known species, that surprises us. We hold nature up as the paragon of ingenuity. Yet compared to what our brains could imagine, the diversity of life on Earth occupies a very small corner. Our alternative universes are full of creatures far more diverse, creative and “out there” than the life here. But most of our imaginary creatures would never work because they would be full of physical contradictions. The world of the actual-possible is much smaller than it first appears.
Indeed, as the complexity of life increases so do the limitations. The limits of chemical bonds and the constraints of thermodynamics matter most for the beginning of life. As proto-life begins to touch more kinds of chemical elements, their atomic constraints are added. As life acquires locomotion, the physics of viscosity, surface tension, dynamic resistance come into play. When organisms add eyes, they add the constraints of spectrum and light transmission. The more senses they add, the more physics they touch, the more limits are embedded.
All these constraints trickle upward and remain in place even as evolution proceeds to make more complexity. Eventually as life surrounds itself with more life, it becomes surrounded by entities exerting their own inherent constraints, so life itself acts as a limit to what is possible.
But while the web of life, and its mechanisms, can negatively constrain possibilities, the apparatus of life can also serve as a positive constraint, pushing life toward certain forms, which is the subject of this next section.
The Positive Constraints of Epigenetics
(continued in the next post…..)


